N95 & KN95 Respirator Mask Available For Your Clinic.

FDA Emergency Use Authorization (EUA-March 24, 2020) for FFP2 rated Filtering Facepiece Respirator Masks:
FDA has concluded based on the totality of scientific evidence available that certain imported disposable FFP2 rated Facepiece Respirators are appropriate for use in healthcare settings by Health Care Professionals as recommended by CDC to prevent wearer exposure to pathogenic biological airborne particulates during FFR shortages resulting from the COVID-19 outbreak
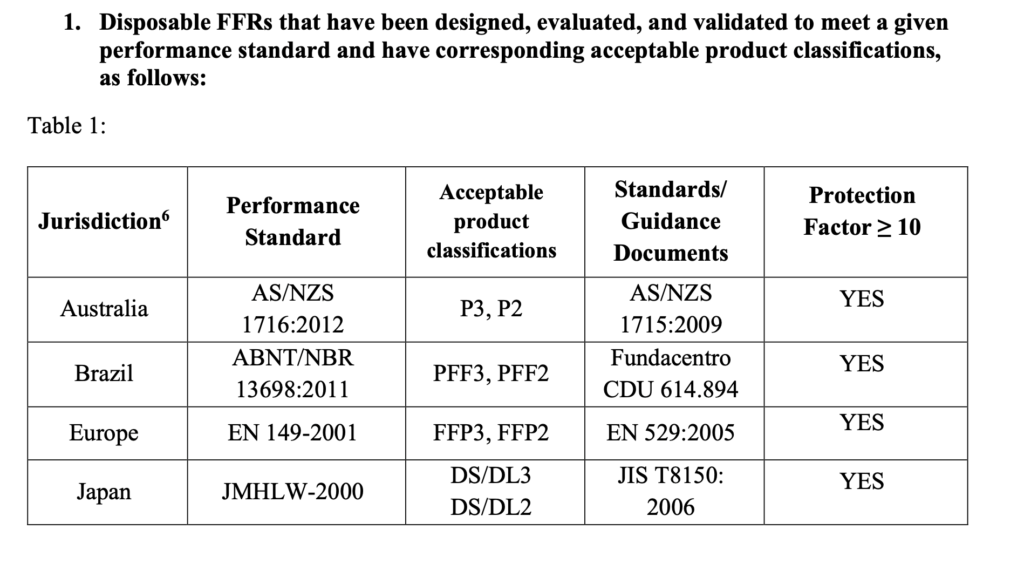
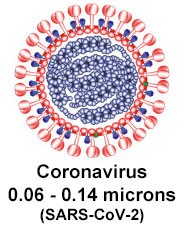
Coronavirus particles range from 60 to 140 nanometers:
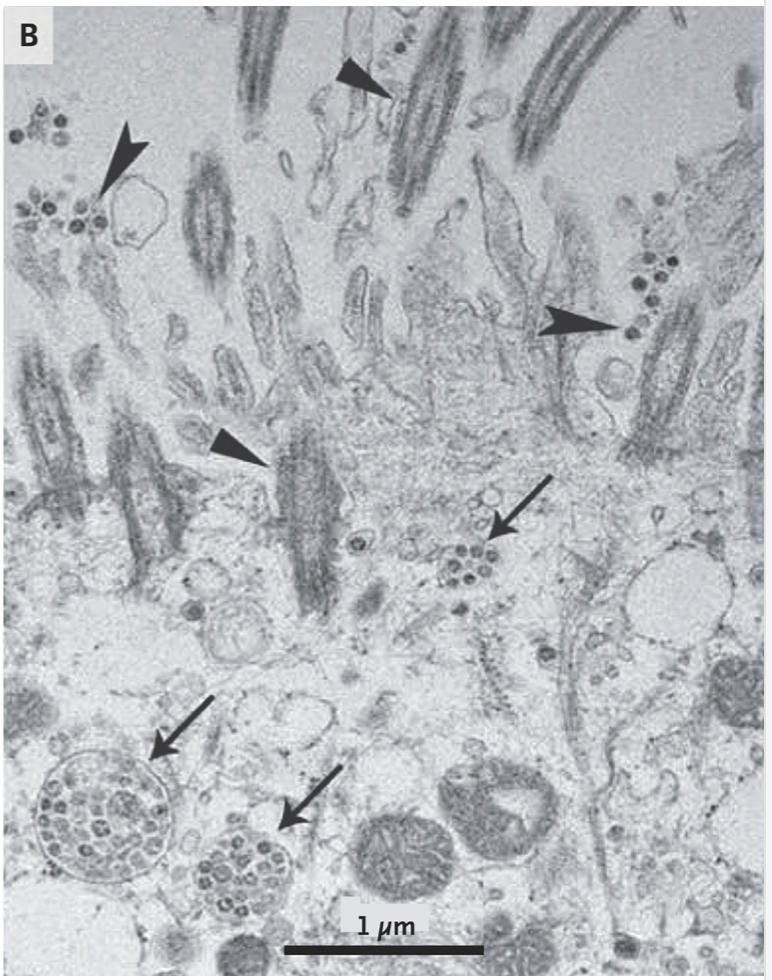
A recent paper “A Novel Coronavirus from Patients with Pneumonia in China, 2019” by Na Zhu, Ph.D., Dingyu Zhang, M.D., Wenling Wang, Ph.D. et al shows that the coronavirus virus particles ranges between 60 to 140 nanometers in size and have distinctive spikes, 9 to 12 nanometers, that give the virion the appearance of a solar corona. Cytopathic effects were observed 96 hours after inoculation on surface layers of human airway epithelial cells
Which kinds of masks and respirators can filter 2019-CoV-2?

Droplets produces by humans by coughing and sneezing range in size from 0.5 microns to 10 microns. Mask and respirators are usually rated according to their efficiency at filtering 0.3 microns particles.
PFE (Particulate Filtration Efficiency) is the measure of the efficiency of the masks and respirators in filtering particles passing through it.
Regular surgical masks are loose fitting, covering the nose and mouth, whereas filtering facepiece respirators (FFRs) are tight fitting masks and are designed to create a facial seal.
Respirators provide about 11.5 to 15.9 times better protection than the surgical masks, and they are preferred when when concerns exist about airborne transmission of bacterial and viral pathogens.
(Refer to Particle Particle Size-Selective Assessment of Protection of European Standard FFP Respirators and Surgical Masks against Particles-Tested with Human Subjects, Shu-An Lee, et al. 2016)
Respirators with high efficiency at 0.3 micron particle size (N95/FFP2 or higher rating) can filter particles down to the size of the coronavirus, which ranges between 0.06 and 0.14 microns in size. Here is why:
High filter efficiency at 0.3 micron size also results in high filter efficiency below this size also due to Brownian motion: The particle’s mass is small enough that it no longer travels in a straight line through the air. It interacts with air molecules and moving in an erratic pattern that allows it to become imbedded in the respirator.
Due to Brownian motion, FFP2 rated filtering facepiece respirator masks are able to capture more than 90% of tiny 0.01 micron particles (10 times smaller than the coronavirus), and regular surgical masks can capture more than 60% of these particles.
Available now under the FDA’s Emergency Use Authorization (EUA) issued on March 4, 2020:

HG KN95 Respirator Mask 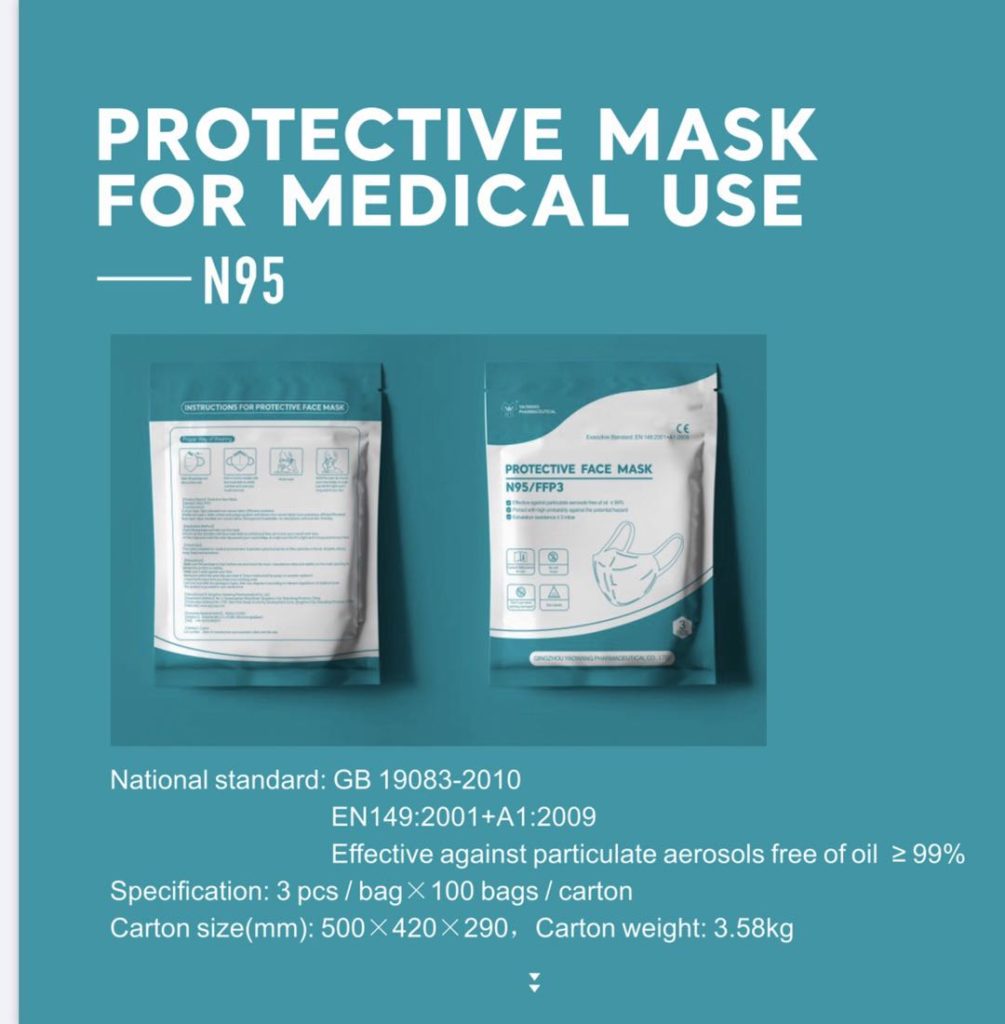
Yao Wang N95 Respirator Mask 
KN95 FFP2-rated Facepiece Respirator Mask
PAGEONE KN95 FFP2-rated Facepiece Respirator Mask

Specifications:
≥ 95% filtration efficiency against solid and liquid aerosols
Lightweight construction for enhanced comfort
The foldable 3-panel design allows for greater facial movement
No Exhale Valve design ( protects both parties)
This mask meets EN149:2001+A1:2009 FFP2 standards for protective equipment
FDA listed
Package:


Dongguan HG KN95 FILTERING FACEPIECE RESPIRATOR (FFR) MASK-FFP2
Performance Standard EN 149-2001 (FFP2).
Authorized by the FDA for use in healthcare settings by healthcare personnel (HCP) when used in accordance with CDC recommendations to prevent wearer exposure to pathogenic biological airborne particulates during FFR shortages resulting from the Coronavirus Disease 2019 (COVID-19) outbreak. ( Pursuant to Section 564(b)(1)(C) of the Federal Food, Drug, and Cosmetic Act (the Act) (21 U.S.C. §360bbb-3(b)(1)(C))
Disclaimer: It is not claimed or suggested that the product is safe or effective for the prevention of COVID-19.
Dongguan KN95-A FFP2-rated Facepiece Respirator Mask Features:

≥ 94% filtration efficiency against solid and liquid aerosols
Lightweight construction for enhanced comfort
Foldable 3-panel design allows for greater facial movement.
Adjustable nose clip provides a secure fit
Dual-strap design to avoid dislodgement.
No Exhale Valve design ( protects both parties)
ISO 9001:2015 Quality Management System
Yao Wang N95 Respirator -A FFP3-rated Facepiece Respirator Mask Features & Package:
Brand Name: Yao Wang N95 respirator mask
Protection rating: FFP3 which is effective against particulate aerosols >=99%.
FDA and CE certificates
Meets EN149:2001+A1:2009 and GB 19083-2010 standards for protective equipment
Package:

Yao Wang N95 & Dongguan HG KN95 are FDA listed and has CE EN 149:2001 Certification:
Tested EN 149:2001+A1:2009 “Respiratory protective devices – Filtering half masks to protect against particles – Requirements, testing, marking”
How to order FFP2 & FFP3-rated Filtering Facepiece Respirator masks for your clinic:
We will process orders for masks and other protective equipment in the sequence that they are received.

